arsenic valence electron|Arsenic Valence Electrons : Baguio 119 rows — Mar 23, 2023 — For main group elements (i.e s-block and p-block elements), the valence electrons are the electrons present in the outermost orbit. But for most of the transition and inner transition elements, the valence . Elephant Bet é reconhecido como número um no mundo das apostas esportivas e jogos de azar na África. A Elephant Bet MZ garante uma experiência de apostas segura e emocionante para seus usuários, contando com a licença N°104/IGJ/JDS/2018 emitida pela Inspeção Geral de Jogos de Moçambique.Desenvolvendo sua atividade à letra da lei, a .
PH0 · Valence Electrons Chart for All Elements
PH1 · How to Find the Valence Electrons for Arsenic (As)?
PH2 · How to Find the Valence Electrons for Arsenic (As)
PH3 · How many valence electrons does Arsenic have?
PH4 · How do you calculate number of valence electrons in an arsenic
PH5 · How Many Valence Electrons Does Arsenic (As) Have?
PH6 · Arsenic valence electrons
PH7 · Arsenic Valence Electrons
PH8 · Arsenic (As)
PH9 · Arsenic
若使用上述奖金计算功能即表示您同意网站使用条款及条件。
arsenic valence electron*******119 rows — Mar 23, 2023 — For main group elements (i.e s-block and p-block elements), the valence electrons are the electrons present in the outermost orbit. But for most of the transition and inner transition elements, the valence .Peb 14, 2014 — Arsenic has 5 valence electrons. It's electron configuration is #1s^2# #2s^2# #2p^6# #3s^2# #3p^6# #3d^10# #4s^2# #4p^3#. It's outermost shell (4s and 4p) has 5 .Nob 28, 2020 — There are two ways to find the number of valence electrons in Arsenic (As). The first is to use the Periodic Table to figure out how many electrons Arsenic h.
Abr 22, 2018 — Arsenic is in group 15, so its atoms have 5 valence electrons. We can also determine the number of valence electrons of the representative elements by determining their .Arsenic is a common n-type dopant in semiconductor electronic devices. It is also a component of the III–V compound semiconductor gallium arsenide. Arsenic and its compounds, especially the trioxide, are used in the production of .January 27, 2021 1 Comment. Get to study the Arsenic valence electrons here to get better in chemistry. We shall disclose every possible information of this element below. Arsenic is one of the most used chemical elements in the .Arsenic is the 33rd element in the periodic table and has a symbol of As and atomic number of 33. It has an atomic weight of 74.92159 and a mass number of 75. Arsenic has thirty-three protons .Mar 17, 2024 — Arsenic has 5 valence electrons. Methods. We can write the valence electrons of arsenic using two different methods: #1 Using periodic table. #2 Using electron configuration. Let’s break down each method in detail. .
Arsenic is a chemical element of the periodic table with chemical symbol As and atomic number 33 with an atomic weight of 74.9216 u and is classed as metalloid and is part of group 15 .
Ene 1, 2015 — The electronic configuration of the stable form of arsenic, As(0), can be written as shown below to illustrate how the presence of five valence electrons allows arsenic to participate in chemical bonding, with an empty p orbital for electron occupation; this can also be illustrated by the electron dot model of arsenic (Figure 1–1).May 19, 2024 — This table of element valences includes the maximum valence and most common valence values in chemistry. Use this for reference with a periodic table. . You may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that can be derived by . Arsenic-3, (+2), +3, +5: 34 .
May 25, 2023 — Arsenic has 5 valence electrons because there are 5 electrons present in the outermost shell of the Arsenic (As) atom. Now let’s see how you can easily find the valence electrons of Arsenic atom (As). If you don’t want .
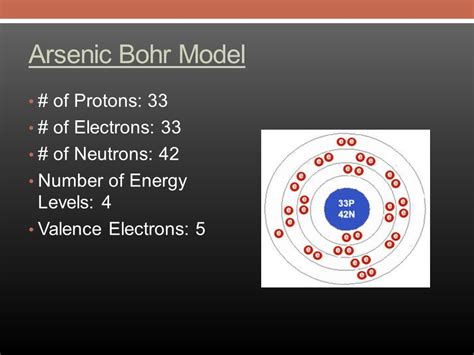
May 1, 2022 — Quels sont les éléments de valence de l’arsenic. Les électrons de valence sont le nombre d’électrons dans la dernière orbite (coquille). La valence de l’arsenic est la somme de tous les électrons de la dernière couche suivant la configuration électronique.
In arsenic acid, arsenic has a valence state of +5, so in addition to the three bonds it forms with the OH groups, it also makes a double bond with the remaining oxygen atom in the formula. . So, again, all of the oxygen atoms are sharing two valence electrons, each of the hydrogens accept one valence electron from oxygen to form a bond, and .
Ago 22, 2024 — Now, the electron configuration of arsenic shows that the last shell of arsenic has five electrons. Therefore, the valence electrons of arsenic are five. The last electron of arsenic enters the p-orbital. Therefore, it’s a p-block element. To know these properties of arsenic one must know the number of electrons and protons of arsenic. ReferenceArsenic is the 33rd element in the periodic table and has a symbol of As and atomic number of 33. It has an atomic weight of 74.92159 and a mass number of 75. Arsenic has thirty-three protons and forty-two neutrons in its nucleus, and thirty-three electrons in four shells. .The electrons that determine valence – how an atom reacts chemically – are those with the highest energy.. For a main-group element, the valence electrons are defined as those electrons residing in the electronic shell of highest principal quantum number n. [1] Thus, the number of valence electrons that it may have depends on the electron configuration in a .Arsenic is a chemical element with the symbol As and the atomic number 33. It is a metalloid and one of the pnictogens, . The valence electron count of GaAs is the same as a pair of Si atoms, but the band structure is completely different which results in distinct bulk properties. [37]
May 1, 2024 — Find your element on the table. Now, locate the element that you want to find the valence electrons for on the table. You can do this with its chemical symbol (the letters in each box), its atomic number (the number in the top left of each box), or any of the other pieces of information available to you on the table.. For example purposes, let's find the valence .Abr 21, 2023 — The second electron will also enter the p y orbital in the clockwise direction and the third electron will also enter the p z orbital in the clockwise direction.. Now, when the fourth electron wants to enter the p-subshell, then it .Nob 13, 2020 — Arsenic is a chemical element with atomic number 33 which means there are 33 protons and 33 electrons in the atomic structure.The chemical symbol for Arsenic is As. Electron Configuration and Oxidation States of Arsenic. Electron configuration of Arsenic is [Ar] 3d10 4s2 4p3. Possible oxidation states are +3,5/-3. Electron Configurationwe can calculate the number of electrons present in the valence shell by knowing the electronic configuration of Arsenic; The electron configuration for the ground-state neutral arsenic atom is given by; 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2 4 p 3 or [Ar] 3 d 10 4 s 2 4 p 3. As, it has 5 electrons in its outermost valence shell (4 t h .

Okt 12, 2020 — These have an AB formula, and there are always 8 valence electrons in the formula. For example, Ga has 3 and As has 5, so GaAs has 8. Another example is ZnSe, (Zn has 2 valence electrons in 4s, Se has 6 valence electrons just like O). Thus, they have the same number of valence electrons as C or Si.Arsenic Valence Electrons Hun 16, 2016 — The complete electron for a neutral arsenic atom is: "1s"^2"2s"^2"2p"^6"3s"^2"3p"^6"3d"^(10)"4s"^2"4p"^3 Its shorthand electron configuration is: ["Ar"]"3d"^(10)"4s"^2"4p"^3 As is the chemical symbol for the element arsenic. Its atomic number is 33, which is the number of protons in the nuclei of its atoms. In a neutral atom, the number .
arsenic valence electron Arsenic Valence Electrons Hun 16, 2016 — The complete electron for a neutral arsenic atom is: "1s"^2"2s"^2"2p"^6"3s"^2"3p"^6"3d"^(10)"4s"^2"4p"^3 Its shorthand electron configuration is: ["Ar"]"3d"^(10)"4s"^2"4p"^3 As is the chemical symbol for the element arsenic. Its atomic number is 33, which is the number of protons in the nuclei of its atoms. In a neutral atom, the number .Ago 3, 2021 — Let us calculate the number of Valence electrons in AsF 3. Arsenic Trifluoride comprises three Fluorine atoms and one Arsenic atom. Arsenic is in group 5 of the periodic table with the electronic configuration [Ar] 3d¹⁰4s²4p³. Therefore, the single Arsenic atom contributes 5 x 1 = 5 valence electrons. Fluorine is a halogenic compound.
Arsenic has 5 valence electrons. It's electron configuration is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 1 0 4 s 2 4 p 3 . It's outermost shell ( 4 s and 4 p ) has 5 electrons, these are the valence electrons.
Ago 5, 2024 — Here we will discuss the direct relation between the electronic configuration of the element and its location in the periodic table. As Arsenic is present in group 15, so it will have 5 valence electrons. Note: The value of n is indicated by .arsenic valence electronAgo 5, 2024 — Here we will discuss the direct relation between the electronic configuration of the element and its location in the periodic table. As Arsenic is present in group 15, so it will have 5 valence electrons. Note: The value of n is indicated by .
Kang Yun-seong; Kang Yoon-seong; Content Score . 100. Yes! Looking good! . Keyboard Shortcuts. Login to report an issue. Kang Yun-sung. Biography. Korean director best known for his 2017 hit film The Outlaws. Korean director best known for his 2017 hit film The Outlaws. Read More . Known For. The Outlaws. Big Bet.
arsenic valence electron|Arsenic Valence Electrons